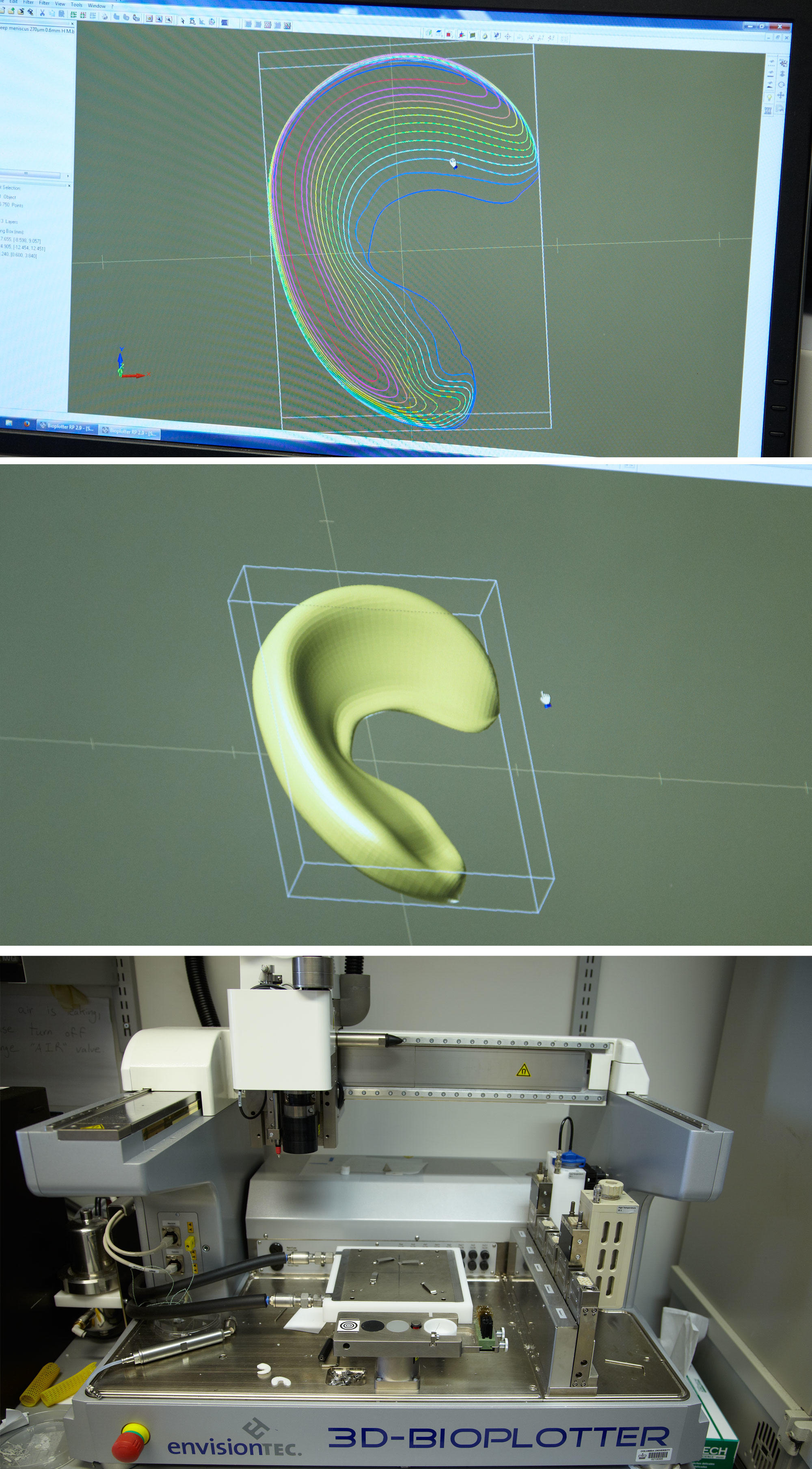
A tiny miracle is taking shape in Jeremy Mao’s laboratory. The needle-tipped nozzle of a 3D printer zips back and forth, shooting out a thin stream of white polymer onto a piece of paper. What emerges, layer by layer, is a one-inch crescent the size and shape of a human meniscus — the tough, fibrous band of cartilage that serves as the knee’s shock absorber. This artificial body part is not intended as a mere replacement. It is something far more remarkable: an ingeniously constructed bridge to healing. Once implanted into an injured knee, it will give the body a foundation for growing a whole new meniscus.
The capacity to heal is one of the human body’s most amazing characteristics. But some parts of our anatomy — including cartilage, tendons, and ligaments in joints — have limited powers of self-renewal. That’s where medical researchers like Jeremy Mao step in. For the past fifteen years, Mao has been at the forefront of the interdisciplinary field of tissue engineering, which combines elements of cell biology, genetics, materials science, and biomedical engineering, all in hopes of enhancing the body’s regenerative powers. Mao, who trained originally as a dentist and oral surgeon in his native China, was drawn to tissue engineering because he thought that certain craniofacial conditions, such as painful disorders of the temporomandibular joint (TMJ), could be treated by coaxing a patient’s stem cells into repairing worn-out bones and cartilage in the jaw. His work at Columbia’s College of Dental Medicine, where he is the Edwin S. Robinson Professor of Dentistry and codirector of the Center for Craniofacial Regeneration, continues toward that ambitious goal; in recent years, however, Mao’s research has broadened to include the regeneration of bone, joint, and soft tissues throughout the body.
“People familiar with my tissue-engineering work are often surprised to hear that I’m a dentist,” says Mao. “Dentistry, I remind them, is about much more than teeth. A great deal of reconstructive and plastic surgery is performed on the mouth. It’s an intricate and complex region — similar to the knee.”
Mao’s latest project is intended to address one of the most common of all human-joint injuries. Every year, hundreds of thousands of people in the US — and millions around the world — tear a meniscus, typically in a sports-related mishap or as a result of cumulative wear and tear. Many people just live with the injury, whose effects can include chronic pain, swelling, and instability. Approximately 750,000 a year in the US opt for surgery. Depending on the severity of the injury, a surgeon may suture the meniscus or remove it altogether. Many patients, regardless of the treatment, eventually develop arthritis in the affected knee.
“Artificial implants exist, but they have serious drawbacks and aren’t used very often,” says Mao, referring to synthetic replacements that are available in Europe and may soon be approved for use in the US. “The main shortcoming of an artificial implant is that it doesn’t mesh perfectly with what’s around it. So it might cause irritation to the surrounding tissues, it might prove unstable, and it might wear out even faster than the original meniscus and then need to be replaced again.”
A new meniscus grown from a patient’s stem cells, on the other hand, would be a genetic fit for that person — a true example of personalized medicine. “Maybe, just maybe, it will fit into the body just as nicely as the original,” says Mao. “This is the promise of tissue engineering.”
Mao, who leads an interdisciplinary team of about twenty scientists and engineers at Columbia University Medical Center, began looking for a way to regenerate a meniscus about five years ago. The basic approach he chose was one that many tissue engineers were already using in their fledgling attempts to help the body regrow bits of damaged bone, skin, and soft tissue: this was to fabricate an implantable scaffold upon which new tissue could take shape. Using a special high-resolution 3D printer, Mao and his colleagues made a meniscus scaffold out of a biocompatible material called polycaprolactone, which is also used to make surgical sutures. The scaffold was honeycombed with tiny interior channels, into which the scientists could inject a variety of biological ingredients to stimulate the growth of new tissue.
“The idea was for new tissue to grow throughout the scaffold, which would slowly dissolve,” says Mao. “It took us months to determine the optimal size and shape of the interior channels. The surface geometry had to be just right for cells to take root.”
But the most difficult challenge for any tissue-engineering project is identifying the proper ingredients to stimulate growth. This is where Mao and his colleagues proved to be innovators. At the time they began working on the project, most tissue engineers thought it was necessary to populate a scaffold both with a patient’s stem cells, harvested from bone marrow, and with proteins called growth factors, which tell stem cells how and when to form different types of tissue. Scientists would typically let these ingredients incubate in a scaffold before implanting the device so that a thin veneer of laboratory-grown tissue would envelop it first.
“The number-one obstacle you face is the host’s body rejecting newly engineered tissue,” says Mao. “You have to get over that initial hump for the long-term benefits of tissue engineering to pay off. And the way to do that — the thinking has always been — is to do as much of nature’s work as possible in your laboratory ahead of time.”
Mao’s team discovered a better approach. Just a year before the researchers began work on the meniscus, they successfully regenerated damaged cartilage in the joints of rabbits using scaffolds seeded not with stem cells or in vitro flesh but a single protein. With just the right protein in a scaffold, the researchers showed, the animal’s body would accept the device and dispatch its stem cells to build the desired tissue from scratch.
“This was really a major breakthrough, promising a quicker, safer, and more reliable method of tissue engineering,” says Francis Y. Lee, a professor of orthopedic surgery and director of the Center for Orthopaedic Research at CUMC. Among the potential benefits of Mao’s approach, Lee says, is that it could generate functional tissue in less time than conventional tissue-engineering techniques. This is because it doesn’t require harvesting and culturing a patient’s stem cells in a laboratory, which can take several weeks or even months. Furthermore, Mao’s approach eliminates the risk of a patient’s stem cells being contaminated in a lab, which can disrupt the new tissue’s development and lead to its being rejected by the body. Says Lee: “Clearly, anything we can do to make the process more patient-friendly will push the field forward and be good for medicine.”
But was Mao’s approach suitable for larger mammals? Last year, he approached Scott Rodeo, an orthopedic surgeon at New York City’s Hospital for Special Surgery, and Lisa Fortier, a veterinary scientist at Cornell University, to conduct a trial of his meniscus-regeneration technique on sheep. At Cornell’s veterinary hospital, several sheep underwent a procedure that Mao has long envisioned providing for people. First they each had an individually tailored 3D scaffold created for a new meniscus. Next, two growth factors were embedded in the scaffold. Finally, the devices were surgically implanted in the animals.
“Then you watch carefully — for months,” says Mao. “Will the scaffolds be rejected? Will the sheep show discomfort? Will their knees be unstable?”
This past winter, Mao, Fortier, Rodeo, and three colleagues in Mao’s lab published a paper in the journal Science Translational Medicine showing that the 3D-printed scaffolds performed beautifully in sheep. The first animals they treated developed normal-looking meniscuses within three months. A video on the journal’s website shows the sheep putting weight on all four legs and scampering around without a limp.
This year, after making minor adjustments to their technique, the researchers successfully operated on several more sheep. Based on the positive results, Mao and Rodeo are now seeking FDA approval to try out the procedure on human patients, which they are hoping to do within the next year or two. Mao says that he is already receiving e-mails and phone calls from people eager to participate in a trial. Many of them have seen news reports about his team’s progress; others have heard through patient networks that a novel solution to their chronic pain may be on the horizon.
“It’s been a reality check for me to realize how much clinical need there is out there,” says Mao. “Patients like the idea of getting a replacement body part that is natural, and genetically their own.”
Ultimately, Mao hopes the techniques that he and his collaborators are developing will prove useful to researchers trying to regenerate other body parts. His team is already experimenting with the design of more-complex tissues, like teeth, arm and leg bones, and muscles and bones that could be used to reconstruct the faces of people injured in catastrophic accidents.
“We want to apply our discoveries to as many medical conditions as possible,” Mao says, holding a 3D-printed meniscus. “This small piece of cartilage is just the start.”
Watch a video of Mao discussing his research: