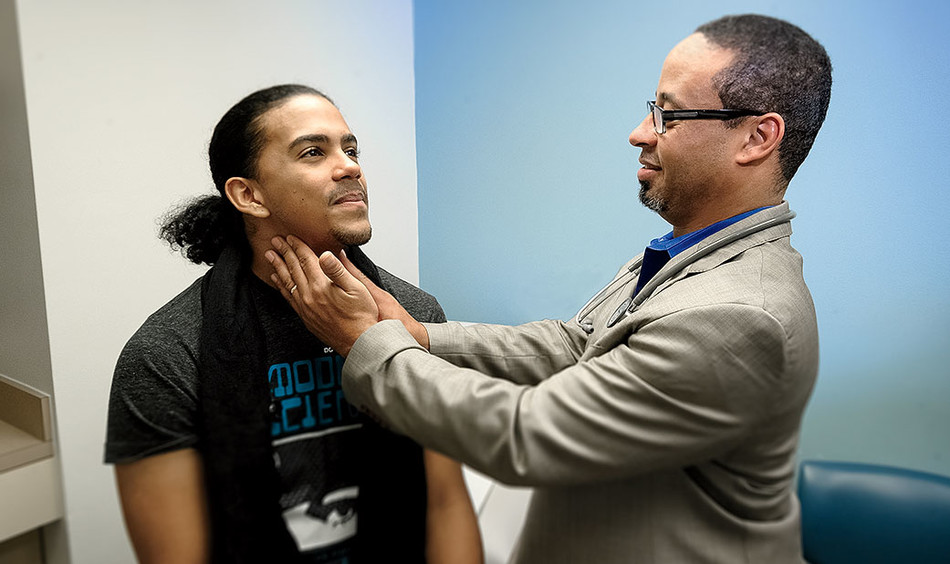Improving diversity among drug-trial participants is a critical step toward reducing racial and ethnic disparities in health, experts say. In the US, 12 percent of the population is Black and 18 percent is Hispanic or Latino, but among the thirty-two thousand patients who participated in clinical trials that led to FDA-approved new drugs in 2020, only 8 percent were Black and 11 percent Hispanic.
Now Columbia University Irving Medical Center (CUIMC) and its Herbert Irving Comprehensive Cancer Center have teamed up with the pharmaceutical company Pfizer to reduce those health disparities by establishing the new Columbia-Pfizer Clinical Trials Diversity Initiative. Pfizer will provide a three-year, $10 million grant to Columbia to support the initiative, which seeks to increase both the participation of underrepresented minorities in medical trials and diversity among clinical researchers.
“A diverse research staff not only helps to improve trust in clinical trials among participants from underserved groups but improves the entire clinical-trial enterprise by bringing different questions, experience, and perspective to the table,” says Anil K. Rustgi, the director of the cancer center and the interim executive vice president and dean of the faculties of health sciences and medicine at CUIMC.