Mention stem-cell research and many people will think of the political controversy surrounding it. From the Bush administration’s veto in July of a bill that would have loosened restrictions on stem-cell research to the election-year commercials featuring Parkinson’s patient Michael J. Fox, public discussion has often focused more on the ethics than on the science.
Medical procedures based on stem-cell research have in fact been conducted since the 1960s, but public awareness didn’t grow until the late 1990s, when James Thompson, a developmental biologist at the University of Wisconsin–Madison, first extracted embryonic stem cells (ES cells) from humans. Thompson’s work provided a way to potentially create a blank canvas, a supply of cells that can mature into nerve, skin, muscle — any of the thousands of kinds of cells that make the body run. Doctors can cultivate stem cells in a dish and can transfer them from dish to dish; these stem-cell colonies, also known as lines, propagate indefinitely. Research flourished in the 1990s, but in 2001, the Bush administration restricted federal funding for research on stem-cell lines cultivated after that point, citing ethical questions of when human life begins.
At Columbia, and elsewhere, however, the science is moving forward. The University has been careful to use private funds for research on newer ES cell lines, and federal funds for work on the older, approved cell lines, with separate facilities and equipment for each.
In 2005 Columbia launched a campaign to raise $50 million for stem-cell research. In May 2006 Columbia announced two initiatives: a research collaboration with the Harvard University Stem Cell Institute and the New York Stem Cell Foundation (NYSCF), a nonprofit dedicated exclusively to accelerating stem-cell research and the establishment of a privately funded laboratory by Project ALS. The goal of the Columbia-Harvard-NYSCF collaboration is to create disease-specific stem-cell lines, beginning with Type 1 diabetes. This project was the first to take place in NYSCF’s privately funded lab in New York City, which is devoted to human embryonic stem-cell research. The Project ALS/Jenifer Estess Laboratory for Stem Cell Research aims to provide advanced understanding of amyotrophic lateral sclerosis (ALS, or Lou Gehrig’s disease) and other motor neuron disorders.
Along with this progress, however, research institutions face real obstacles. “[Bush’s mandate] has created tremendous barriers,” says Ruth Fischbach, professor of bioethics and director of Columbia’s Center for Bioethics. “We have to be excruciatingly careful to make sure no federal funding is ever used for the privately funded research.” Her husband, Gerald Fischbach, John E. Borne Professor of Medical and Surgical Research (in Pharmacology) and former dean of CUMC, has testified before Congress in favor of stem-cell research, and the two regularly speak on panels about the ethical and scientific aspects of the issue.
Stem-Cell Sources
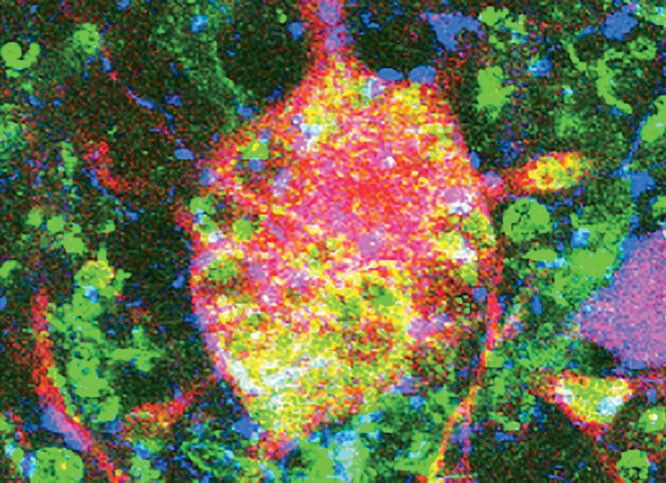
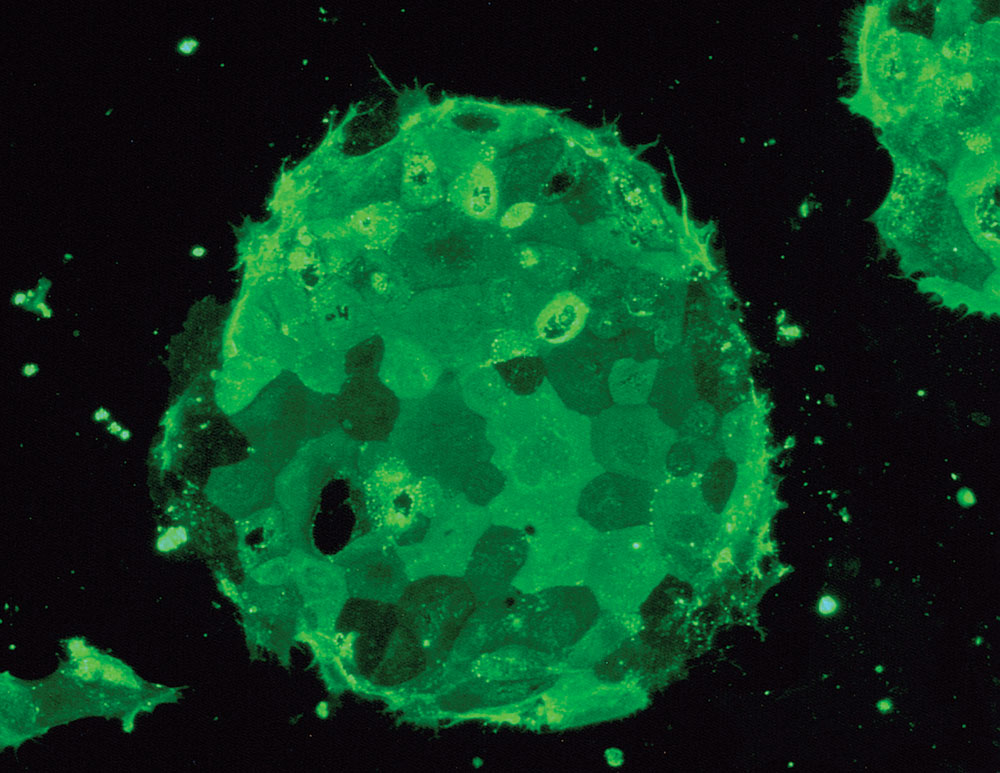
Stem cells are cells that can divide indefinitely, and in some cases can mature into any kind of cell in the body. Researchers have identified three sources of stem cells, among which adult stem cells are the least controversial. These are cells that normally reside in the human body and replace worn-out or damaged cells. In bone-marrow transplants, for example, a donor’s bone marrow, rich in healthy stem cells that can form red and white blood cells or platelets, attaches to the recipient’s bones and begins producing normal cells. While this procedure, first pioneered in the 1960s, has saved millions of lives to date, the use of adult stem cells has several limitations. First, they can only differentiate into the cell type of the source organ. Second, scientists haven’t yet been able to identify them in all organs. Lastly, adult stem cells may not be optimal for research, having been exposed to the various toxins and strain typically endured in the course of a human life.
Embryonic stem cells, on the other hand, are pure, and can potentially develop into any kind of cell. These are derived from embryos donated to science by couples who have undergone in vitro fertilization — not from aborted fetuses, as some may assume. “ES cells are not taken from abortion clinics,” notes Hynek Wichterle, an assistant professor of pathology at CUMC and member of the Columbia Center for Motor Neuron Biology and Disease (Motor Neuron Center/MNC), who uses stem-cell research to study amyotrophic lateral sclerosis (ALS, or Lou Gehrig’s disease). “They are embryos taken five days after fertilization, before a woman would actually know she is pregnant.” These microscopic balls of cells, called blastocysts, may not even have made their way down the fallopian tubes, much less taken root in the uterus.
A third source of stem cells is somatic-cell nuclear transfer, commonly known as cloning. Its use in stem-cell research is not as widespread. The procedure has been shown to work in mouse cells, but scientists are still working on developing reliable methods to apply it to human cells. Researchers extract the nucleus of an adult cell, perhaps from a skin biopsy, and inject it into an embryonic cell whose nucleus, or DNA, has been removed. From there they have a fresh supply of ES cells. The benefit of cells derived this way is that scientists can take cells from a patient who has a disease and create ES cells that have the genetic makeup of someone with the illness, and thus use them to study how the disease works and its response to different drugs.
Building Better Neurons
Amidst the pipettes, microscopes, and machines in his lab on 168th Street, Asa Abeliovich, assistant professor of pathology at CUMC, is trying to uncover why dopamine neurons die in the brains of people who have Parkinson’s disease. Dopamine is a chemical that transmits messages to the parts of the brain that regulate movement and coordination. The loss of cells that secrete dopamine triggers the onset of Parkinson’s symptoms. Scientists have developed therapies that can temporarily relieve some symptoms, but they have yet to find treatments that can actually prevent or inhibit the loss of dopamine neurons. “It’s just striking that none of the drugs have any impact,” says Abeliovich. “Disease progression is inexorable.”
Doctors first identified Parkinson’s in the early 19th century, but they have yet to find a cure. Abeliovich uses ES cells and manipulates them in a petri dish to mimic the development of dopamine neurons. He hopes eventually to cultivate healthy neurons for injection into a sick patient. His work bridges the gap between developmental neuroscience and clinically relevant research.
At CUMC’s Motor Neuron Center (www.columbiamnc.org), Christopher Henderson, Wichterle, and Thomas Jessell are researching ALS and spinal muscular atrophy (SMA), a disease that is also caused by the death of neurons, in this case, motor neurons. Motor neurons are responsible for transmitting messages from the brain telling muscles how to move. When ALS strikes, the messages that tell muscles to contract stop. That is how the Iron Horse, Lou Gehrig, who attended Columbia College from 1921 to 1923 before joining the New York Yankees, lost his legendary endurance. In 1939, at age 36, he was diagnosed with ALS. His deterioration was rapid. He lost movement, his muscles weakened and atrophied, and soon he couldn’t swallow or speak. Within two years, he was dead. The majority of patients with ALS die from respiratory failure within five years of diagnosis.
While ALS mainly afflicts people between the age of 40 and 70, SMA is more common in children. Both are neurodegenerative diseases for which stem-cell research offers tremendous promise. In 2002 Wichterle showed that mouse ES cells could be manipulated to become motor neurons. Among ALS cases, 10 percent are hereditary. For those cases, Henderson and his colleagues are deriving motor neurons from ES cells from blastocysts donated by couples whose in vitro embryos were found to carry the gene mutation characteristic of ALS. “Our goal,” says Wichterle, “is not to derive new embryonic stem cells that are normal, but to derive cells that carry the same mutations in patients that have motor neuron diseases.” With that ability, Wichterle hopes to understand how the disorder works, and to test drugs that could slow or even halt the dying of motor neurons.
For the 90 percent of ALS cases that are nonhereditary, or “sporadic,” research with ES cells derived from somatic-cell nuclear transfer hold greater potential. Wichterle and his colleagues are working on ways to take skin cells from ALS patients, inject the nuclei into embryonic cells in which the DNA has been removed, and then derive new ES cells that carry the DNA of patients with ALS. Henderson is quick to point out that the cloning process is far from the image of Dolly the sheep that comes to mind. “We are not grafting cells,” he says. “These ES cells stay in the culture dish, and they should be a tool for drug discovery and understanding of the disease.”
Hearts and Eyes
According to the National Heart Lung and Blood Institute, more than a million people in the U.S. suffer from heart attacks each year. Half of them die, and half of those within an hour of the attack. Women are more likely to die within a few weeks of the attack than men. Silviu Itescu, associate professor and director of transplantation and immunology in CUMC’s department of surgery, uses adult stem cells to help fight heart attacks, the number-one killer of men and women.
In the 1990s, Itescu made a key finding, identifying endothelial stem cells — that is, blood-vessel-forming cells — in bone marrow. When transplanted back into a patient, the cells could stimulate blood-vessel growth and in turn supply much-needed oxygen to the heart. But the procedure requires that the cells come from a matching donor, or else the risk of rejection is high. Endothelial cells also can’t be grown in large quantities in a culture dish.
Mesenchymal cells may provide an alternate solution. Also found in bone marrow, these cells are far more versatile than endothelial cells. Not only can they mature into blood vessels, but they can differentiate into bone, cartilage, and fat cells. They also have a much lower risk of immune rejection, so doctors don’t have to find matching donors. “Unlike endothelial cells, you can put mesenchymal cells into culture and grow them from initial cells, generating hundreds of millions, even billions, of cells,” says Timothy Martens, an associate research scientist in Itescu’s lab.
Itescu and Martens, along with Eric Rose, who is the Morris and Rose Milstein, Johnson & Johnson Professor of Surgery at CUMC, are working on developing a versatile supply of cells that can be injected into patients who have just had a heart attack, regardless if they match the donor. “This type of approach has the potential to fundamentally change therapeutic outcome in cardiac medicine,” says Itescu.
One area of stem-cell research in which cell replacement has already been put in use is ophthalmology. According to Stephen Tsang ’96GSAS, ’98PS, assistant professor of clinical ophthalmology, stem-cell therapy is a routine treatment for severe alkaline burns (which result from household or industrial accidents). In this case, limbal stem cells reside between the clear and white part of the cornea and the white part of the eye, and are responsible for maintaining the cornea’s transparency. After an alkaline burn, those stem cells are damaged, and the cornea loses its transparency and turns opaque. Once the inflammation dies down, doctors take limbal stem cells from the patient’s healthy eye and graft them onto the damaged one.
Now Tsang and his colleagues hope to apply similar methods to macular degeneration, a disease in which a part of the retina deteriorates. It is the leading cause of legal blindness in people over the age of 60, affecting at least 15 million Americans. Twenty percent of cases are in patients between ages 65 and 75. “Baby boomers are in their 60s now, but in ten years macular degeneration will become endemic,” says Tsang. The effects are far-reaching. Many patients become depressed because they can’t read or leave the house, people get injured from falling, and the risk of heart disease increases because of a lack of exercise.
Experiments with goldfish have shown that they can heal their own damaged retinas by regenerating neurons from stem cells taken from the ciliary body, which is located at the front of the eye behind the iris. Tsang is currently testing the same procedure on mice. He will take ciliary-body stem cells, differentiate them into retina neurons, and transplant them into the back of the eye.
A Growing Field
For most diseases, stem-cell research is very much in its infancy, and it will take years before many researchers see their work translate into clinical applications, as Stephen Tsang has. But Columbia scientists are making the push. For example, Rudolph Leibel, professor of pediatrics and medicine, and co-director of CUMC’s Naomi Berrie Diabetes Center, hopes his work with ES cells derived from somatic-cell nuclear transfer will lead to a better understanding of how the pancreas develops. René Hen, professor of pharmacology, studies adult stem cells in the hippocampus in his work to discover a new depression therapy. Fiona Doetsch, assistant professor of pathology, is trying to identify neuron-producing stem cells in the adult brain.
Asa Abeliovich hopes that as research progresses, the skepticism and controversies will lessen. He compares the furor that surrounds stem-cell research to the flap over recombinant DNA engineering in the 1970s, when scientists first manufactured insulin from human DNA. “The idea was very contentious 30 years ago, but as people came to see the benefits, they became more comfortable with it,” he says. “If we can develop stem-cell therapies for Parkinson’s that are very effective, and show in very plain terms what progress has been made, it’s going to be clear where the balance falls.”
Tempest in a Test Tube
Shortly before the 2006 midterm congressional elections, Michael J. Fox made a television appearance that was hard to forget. In a campaign ad in which he appealed to Missouri voters to support the Democratic candidate, who was in favor of stem-cell research, Fox’s symptoms of Parkinson’s disease were so pronounced that Rush Limbaugh accused him of acting and grossly imitated his tremor, which he later apologized for after a storm of protests. State legislation is crucial in the stem-cell debate, since President George Bush restricted federal funds for research that uses embryonic stem cells (ES cells) created after August 2001. “At its core, this issue forces us to confront fundamental questions about the beginnings of life and the ends of science,” Bush said in a speech announcing his decision at that time. “It lies at a difficult moral intersection, juxtaposing the need to protect life in all its phases with the prospect of saving and improving life in all its stages.”
In July 2006, Bush issued his first veto, voting against a bill proposed by Congress to loosen the restrictive mandate. States including California, Connecticut, and New Jersey have passed legislation to approve state funds for ES cell research. “Bush managed so cleverly to balance on the edge of a knife blade,” says Ruth Fischbach, professor of bioethics and director of Columbia’s Center for Bioethics. “In his heart of hearts he perhaps may feel that there is a future in stem-cell research, but he was very influenced by the religious right, and he had to make this compromise.”
ES cells are derived from five-day-old blastocysts from embryos that are in excess of clinical need from in vitro fertilization clinics. In the United States, there are an estimated 400,000 embryos stored in the freezers of in vitro fertilization clinics. Once their embryos are frozen, couples have the option of storing them indefinitely, putting them up for adoption by other couples, discarding them, or donating them to science. Scientists generally hold the view that embryonic research can be conducted on embryos until the 14th day, when the nervous system begins to develop and the embryo is embedded into the placenta — the point at which the embryo, in essence, becomes an individual.
Researchers argue that Bush’s approved existing ES cell lines are inferior and may be contaminated. And, in the five years since Bush’s ruling, scientists around the world have made great progress with embryonic stem cells, developing technology that can’t be applied to the older cell lines. The colonies also don’t offer a large enough sample. “If you want to do research on any subject, you want as wide a range of cell lines as possible,” says Thomas Jessell of CUMC’s Center for Neurobiology and Behavior. “You don’t want to be constrained by a limited number of cells.” In order to create and conduct research on new cell lines, institutions, including Columbia, have had to seek private funding.
While the two sides of the argument are polarized, there is a middle ground of people who believe that even if life begins at conception, scientific research on an embryo that may otherwise be discarded is a worthwhile sacrifice, considering the number of lives stem-cell research could help. “Some people believe that an embryo is a potential life, and they weigh that against the real life of a child with diabetes, or a person paralyzed due to a spinal cord injury, or someone with Parkinson’s disease where, using stem cells, the hope is that we will develop a cure for those disorders,” says Fischbach. “It all depends on your perspective.”