To control global warming, many scientists say we need to minimize the amount of carbon dioxide that coal factories spew into the air, in addition to developing cleaner forms of energy over the long term. This requires inventing a method to vacuum carbon dioxide from factory smokestacks or straight out of the atmosphere, a challenge that Columbia geophysicist Klaus Lackner, among others, is tackling
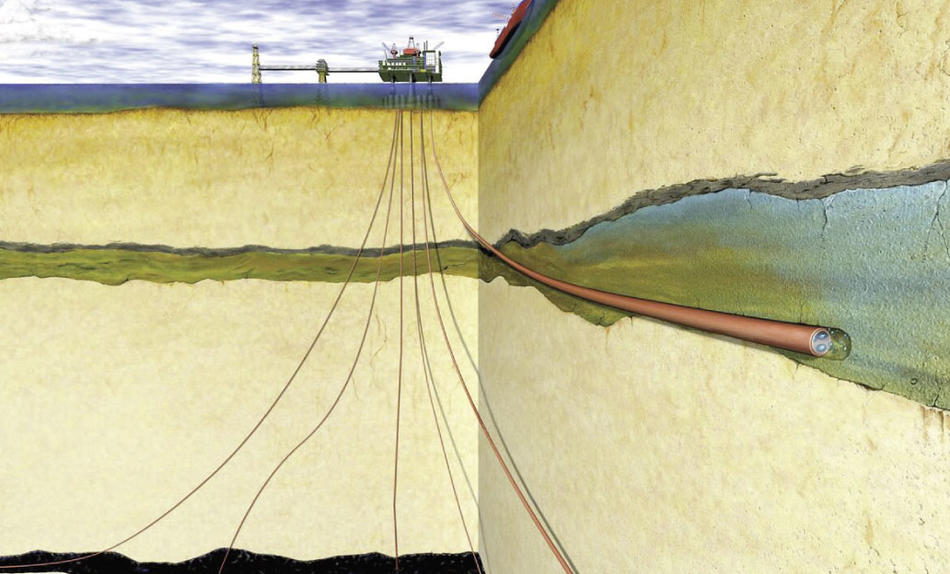
How could we store the captured CO2? That’s another tricky problem, and one that Columbia geophysicist David Goldberg and several colleagues think they have solved. They’ve located huge honeycombed basalt formations 9000 feet underwater off the West Coast of the United States. Liquefied CO2 could be pumped into these watery hollows and remain locked away for good, the scientists say. By analyzing data from previous deep-sea drilling experiments off California, Oregon, Washington, and British Columbia, they estimate that about 30,000 square miles of basalt caverns exist there — enough to hold 150 years’ worth of U.S. CO2 emissions.
Burying CO2 isn’t a new idea. A separate research team headed by Columbia geochemist Juerg Matter is preparing to pump liquefied CO2 into an underground basalt formation at a power plant near Reykjavik, Iceland, in the first such large-scale demonstration of the technique. Columbia scientists have previously shown that when CO2 is combined with basalt, which is hardened lava, the two naturally react to create a solid carbonate — essentially, chalk.
Undersea basalt caverns might be bigger and safer than caves beneath solid land, Goldberg says. If CO2 is buried deep below the ocean, he says, any CO2 that doesn’t react with the basalt won’t be able to rise to the surface and reenter the atmosphere, in part because it’s heavier than water. Underwater basalt formations are “immense, accessible, and well sealed—a huge prize in the search for viable options,” says Goldberg.
He and his collaborators, Taro Takahashi, a geochemist and senior scholar at Columbia’s Lamont-Doherty Research Observatory, and Angela Slagle, a marine geologist and postdoctoral student at Lamont-Doherty, hope the U.S. government will fund studies to determine whether pumping CO2 underwater is economically feasible.
The findings appeared July 14 in the Proceedings of the National Academy of Sciences.