Can We Stop the Next Pandemic?
It was the week when Iceland’s first mosquito was discovered and when authorities confirmed that a Long Island resident had contracted the mosquito-borne and typically tropical chikungunya virus; when an outbreak of Rift Valley fever (mosquitoes) killed dozens of people in West Africa; when Missouri reported three deaths for the year from West Nile virus, California identified three cases of mpox, and Arizona and Utah counted more than a hundred cases of measles; and when influenza was spiking in Japan and Taiwan and climate denial, anti-vaccine sentiment, and funding cuts for public health were endemic in Washington. Which is to say, it was just another week in the fall of 2025 and as good a time as any to ask Ian Lipkin, renowned “virus hunter” and epidemiologist at Columbia’s Mailman School of Public Health, what keeps him up at night.
On the top floors of the Allan Rosenfield Building at Haven Avenue and West 168th Street, Lipkin presides over the Center for Infection and Immunity (CII), which he founded in 2008 to track infectious diseases, identify pathogens, and trace the virological causes of chronic illnesses. CII scientists have worked in hot zones all over the world and identified more than 2,500 viruses.
“Our focus is always on: Why are people sick?” Lipkin says. “What is the agent causing the sickness? How can we detect its presence, and what insights can that give us into what drugs might be useful and what vaccines should be made?”
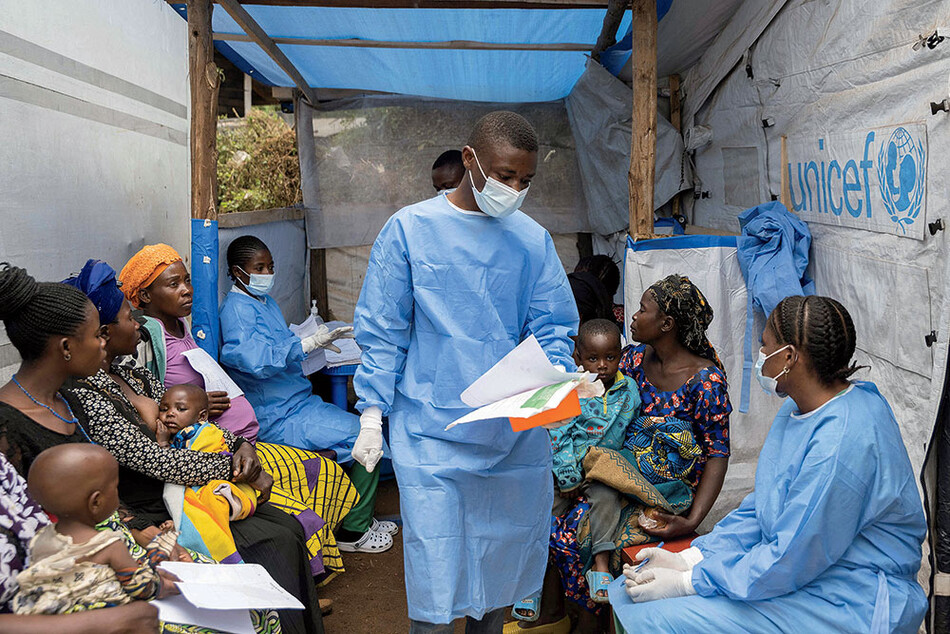
To help answer these questions, Lipkin in 2015 introduced an innovative technique in virus diagnostics. Called the virome capture sequencing platform for vertebrate viruses, or VirCapSeq-VERT, the method can quickly identify any virus that infects mammals, birds, fish, reptiles, or amphibians. Almost immediately, Lipkin began thinking about how to efficiently bring VirCapSeq-VERT to the places — mainly in Africa and Asia — where most viruses emerge. This was public-health common sense, since viruses readily cross borders and oceans, but it also spoke to Lipkin’s commitment to his ethical obligations as a medical doctor. In 2019 — wanting, as he says, to “teach people to fish” and build diagnostic proficiency and independence — Lipkin conceived of the Global Alliance for Preventing Pandemics, or GAPP, a training program that would teach clinicians around the world how to use CII’s signature diagnostic tool.
But just as Lipkin was getting GAPP off the ground, a new respiratory virus appeared in Wuhan, China. The virus, which caused coronavirus disease 2019 (COVID-19), killed thousands of people in Wuhan and spread to every continent. By the spring of 2020, New York City had become the first US epicenter of the pandemic, recording hundreds of deaths each day. Lipkin, locked down in Upper Manhattan, plowed ahead with GAPP. What he needed was the right person to run it.
Around that time, fifty miles north of the city, biochemist Ken Wickiser, a tenured professor and associate dean of research at the US Military Academy at West Point, was torn. A former Army pilot, Wickiser was focused on keeping West Point operational during the pandemic. But the images of the mobile morgues in New York were like a call to action. “I thought, I need to be in this fight,” he says. He had heard about Lipkin’s idea of teaching the VirCapSeq-VERT method to people in virus-vulnerable regions and could think of no better way to respond to a pandemic than to stop the next one. “So I contacted Ian and said, ‘Let me do this. Let me lead GAPP,’” Wickiser recalls. “And he did.”
Wickiser arrived at Columbia in 2021, and he and Lipkin crafted a training curriculum. Wickiser then hired Jack Collins, a student intern at GAPP, as a lab technician and technical instructor and recruited Samuel Yingst, an American veterinary microbiologist based in Cape Town, South Africa, to manage international projects. (Yingst and Wickiser had overlapped as cadets at West Point but didn’t know each other.) With funding from the Skoll Foundation, a philanthropic institution headed by eBay’s first president, Jeff Skoll, as well as grants from the National Institutes of Health (NIH), GAPP held its first training session in 2021 and was poised to play a crucial role in virus detection and global public health.
Viruses are submicroscopic particles consisting of genetic material (DNA or RNA) encased in a protein shell. They proliferate — and make us sick — by invading a host cell, hijacking its machinery, and replicating itself. The difficulty with identifying a pathogen in a given diagnostic sample — blood, mucus, urine, feces, tissue, or wastewater — is that viruses are minuscule in size and number in proportion to the host material (DNA, cells, bacteria). In traditional diagnostic methods, the entire sample is analyzed, including the hundreds of millions of irrelevant gene sequences. That makes virus identification arduous and time-consuming — and that’s where Lipkin’s method comes in.
VirCapSeq-VERT separates out the viruses by harnessing the biological law that a single strand of DNA will always bond with a complementary strand. In Lipkin’s analogy, VirCapSeq-VERT acts like a magnet that draws needles from a haystack. The key to the method is the process of “capture enrichment,” in which clinicians use synthetic, single-strand DNA and RNA virus “probes” to attract the targeted viral DNA and RNA in the sample. Once they isolate the viral strands, they fragment them, “amplify” or copy them, and give each strand a unique DNA “barcode” for identification. Then they place them on a glass slide and feed them into a machine called a sequencer, which reads the order of a strand’s DNA bases (adenine, thymine, cytosine, and guanine) and expresses this code as a string of letters (A, T, C, G). Because the sequencer reads only the viruses and not the other genetic sequences in the sample, it takes hours instead of days.
The clinicians then run the lettered sequences through a database to determine whether the pathogen is a known virus, a variant, or something entirely new — giving them a better chance at keeping it from spreading.
“We’re trying to identify a virus at the early stages,” explains Collins, “before it ever becomes a pandemic.”
GAPP has taught the VirCapSeq-VERT method to clinicians from Africa (Mali, Zambia, Liberia, Kenya, Zimbabwe, South Africa, Nigeria, the Democratic Republic of the Congo), Asia (Bangladesh, Sri Lanka, Taiwan), Europe (the UK, the Netherlands, Germany), and the Americas (Mexico, Ecuador, Nicaragua). “I don’t care where we go,” Lipkin says, “as long as we can figure out the origin of a problem and help people solve it.”
“I don’t care where we go,” Lipkin says, “as long as we can figure out the origin of a problem and help people solve it.”
For the first couple of years, GAPP brought over public-health counterparts in Africa for a three-week training program at Mailman. Then, in 2023, GAPP began going abroad: Yingst, from Cape Town, would assess the labs in partner countries to let his New York colleagues know what was needed and what to expect, and then Collins would fly over and do the training. In 2025, for instance, Collins traveled to the Democratic Republic of the Congo (DRC), a country of 109 million people in Central Africa, where scientists were trying to get a handle on the mpox outbreaks that began in 2022 and the perpetual threat of an Ebola outbreak (there have been sixteen in the DRC since the virus was discovered in 1976).
In a lab in the capital city of Kinshasa, the GAPP team tested different types of samples to see if there were any other viruses circulating that caused a similar type of disease to mpox. Such surveillance is critical: in any outbreak, people get sick from other pathogens, but without good diagnostics, their symptoms may be wrongly attributed to the dominant illness. That means many patients don’t get proper treatment — and many die.
“The DRC commonly has outbreaks of unknown origin,” says Collins. “There have been a couple lately that have required the World Health Organization to respond. They will collect samples of what they suspect is a viral hemorrhagic fever, and we’ll do testing for the most likely and concerning viruses, such as Ebola and Marburg. And if those test negative, we can use our method for investigating other potential viral causes that might not be suspected.”
In Mali, a West African country of twenty-three million people, GAPP has helped scientists sequence samples that tested positive for the dengue virus (mosquitoes) and analyze the geographic and temporal transmission of different dengue virus types throughout West Africa.
“We go to places that other people either aren’t invited to or are afraid to go, whether due to political instability or health risks, and Mali is one of them,” Wickiser says. “Mali suffered a huge dengue outbreak and many people died, including one of our collaborators. But the people there are completely trustworthy, top-notch clinicians and scientists, and they have taken what we have taught them and made it so routine that they can train more and more people.”
GAPP wants desperately to continue this progress, but Lipkin has had to scale back. In March 2025, the Trump administration canceled $400 million in federal research grants and contracts to Columbia. The Mailman School relies on these grants, and Lipkin had to let go a quarter of his staff of highly trained virologists. He publicly warned that without government grants, Columbia would not survive as a research institution — an untold cost that weighed heavily on the University community.
In the end, Columbia negotiated with the government to restore funding, but Lipkin says that CII has recovered only 60 percent of what it had: not all the federal money has returned, and private funding has also been stretched thin as demand has grown.
“So many of the resources that we’ve counted on have dried up — from NIH, from CDC, and from philanthropy,” Lipkin says. “We’re not whole.”
“Before, we never said no to people,” says Wickiser. “Now we have to say no. It’s hard and heartbreaking. People are dying, but I can’t send supplies or staff I don’t have.”
Lipkin’s career was shaped by one of the worst viral outbreaks of the twentieth century. In 1981, the Chicago-born Lipkin, who had studied theater and philosophy at Sarah Lawrence College and earned his medical degree at Chicago’s Rush Medical College, was a twenty-nine-year-old neurology resident at UC San Francisco. There he saw men — mostly young, gay, and otherwise healthy — come into the clinic, one after the next, with unexplained symptoms: fevers, respiratory failure, strange rashes, brain tumors.
Many doctors refused to see these men for fear of stigma or contamination. Not Lipkin: Wanting to learn as much as he could, he saw hundreds of patients and identified symptoms that he showed could be treated with plasmapheresis, a procedure in which harmful autoantibodies induced by the infection are removed from a patient’s blood by separating the plasma from the blood cells.
“It took so long to figure out why people were sick, and in the interim, because there were no diagnostic tests, millions of people became infected and millions of people died,” Lipkin says of the HIV/AIDS crisis. “At that time, people were using very cumbersome, relatively slow methods for identification of infectious agents, and it was clear to me that we needed a faster diagnostic tool capable of working anywhere in the world.”
Lipkin had found his life’s work. He got a fellowship in molecular biology and neuroscience at the Scripps Research Institute, where he started using a molecular-biology technique called “subtractive cloning” to isolate viruses. Lipkin would mix two samples of DNA — a control and a target — and remove all the material common to both, leaving only unique single strands in the target sample: the genes, presumably, of the virus. These strands could then be cloned and studied. Lipkin used this method to make key advances in virus isolation, and the Pew Charitable Trusts, taking notice, gave him money to develop his diagnostic tools.
That investment yielded dramatic results. In the late summer of 1999, a cluster of meningoencephalitis cases turned up in the New York City area. There were also a lot of dead birds around, though at first no one made a connection. Ultimately, fifty-nine people fell ill, and seven died. Lipkin, then director of UC Irvine’s Emerging Diseases Laboratory, was intrigued and offered his help. His team analyzed tissue samples from three human victims and found that they had contracted West Nile virus: Mosquitoes had bitten infected birds (themselves infected by mosquitoes) and then passed the virus to humans. It was the first time the virus had been found in the Western Hemisphere. The discovery made Lipkin’s name in the field of epidemiology, and Columbia hired him in 2001.
Lipkin was just settling into his lab at Mailman at the time of the terrorist attacks of September 11, 2001. A week later, envelopes containing anthrax spores turned up at the US Capitol, the first in a monthlong string of mail attacks that would kill five people and sicken seventeen. This triggered a new national fear: bioterrorism. “At that point, there was a big concern that people would weaponize infectious diseases like smallpox,” Lipkin says. Anthony Fauci, then the director of the National Institute of Allergy and Infectious Diseases, called for a biodefense initiative that would bring together the best minds in the country to study the risks. The US government established the Northeast Biodefense Center, a consortium of twenty-eight medical schools and research institutes, including Columbia, Yale, Cornell, Rutgers, and NYU. Lipkin was director for eleven years.
“Billions of dollars went into it, and we are still reaping those benefits, because we wouldn’t have been able to respond as rapidly as we did to SARS or SARS-CoV-2 or mpox or many other things,” Lipkin says. “All the platforms you need to make vaccines, to do diagnostics, were jump-started by this investment.”
Over the next two decades, Lipkin and his team were on the virus front lines. Lipkin was in China in 2003 during the first SARS outbreak, sharing his diagnostic methods to help contain the spread. He tested samples from Angola during the Marburg outbreak of 2004–05 (Marburg is a hemorrhagic fever spread through bodily fluids), and found that cases of malaria were being mistaken for Marburg and going untreated. Another time, in Gorakhpur, India, near the Nepalese border, where thousands of children were dying every year of encephalitis, Lipkin saw sick kids who were crowded two or three to a bed. Everyone assumed the cause would be a viral infection. But Lipkin’s analysis showed that it wasn’t a virus at all: it was a bacterial infection that could be treated with tetracycline.
The deadliest virus Lipkin ever encountered was in Lusaka, Zambia, in 2008. Five people in Zambia and Johannesburg, South Africa, got sick with fever, headache, and muscle pain, and four died within days of the initial symptoms. A CII team led by epidemiologist Thomas Briese quickly identified the new pathogen, now called Lujo virus (for Lusaka and Johannesburg). The team also found an effective treatment that saved the fifth victim — and stopped the outbreak in its tracks.
“We have to enable every country on the planet to be able to quickly identify novel pathogens within their borders, by themselves.”
Now, with CII’s VirCapSeq-VERT method, Columbia researchers have an even greater ability to stop deadly pathogens before they spread. Yingst says that VirCapSeq-VERT “is exactly what I always wanted as an Army veterinarian” — he spent twenty-five years tracking pathogens in animals in Central Asia, Africa, the Middle East, and Southeast Asia — “and is pretty much everything that you could ever want in a diagnostic tool.”
Had the tool been available in Wuhan in the fall of 2019, Yingst says, COVID-19 could have been contained. “It was a completely novel pathogen never encountered before,” he says. “So unless you had access to this tool, which would have quickly identified it as a type of coronavirus, you were stuck.”
Yingst, like everyone at GAPP, believes that to prevent pandemics, there must be universal access to advanced detection and sequencing. “We have to enable every country on the planet to be able to quickly identify novel pathogens within their borders, by themselves. Otherwise it’s pointless and silly to imagine that we could control a pandemic.”
So what keeps Lipkin, the virus hunter, up at night? It’s a tantalizing question to ask a scientist who was an adviser on the 2011 medical thriller Contagion and who, over his forty-five-year career, has helped contain or prevent many outbreaks in real life. But that week, Lipkin had other preoccupations.
“What keeps me up is something very different,” he says. “You can probably guess. I’m trying to figure out if there will still be a Voting Rights Act. I’m worried about the fact that we no longer have a balance of powers. We live in a very different world.”
Fair enough. But what about, for instance, the avian flu that’s been in the news — the H5N1 virus — which has appeared in dairy cattle in the US?
“H5N1 has popped up periodically ever since it was first detected in 1997 in Hong Kong,” Lipkin says. “Yes, if you get infected with this virus it can be lethal. Yes, it is probably going to be in animals other than birds. But do I think it’s going to be the next pandemic for humans? No, because the receptors it uses are in the lower respiratory tract, not the upper respiratory tract, which means it doesn’t really have the capacity to spread widely. So this is not the one.”
Not that there’s any shortage of candidates. But as Lipkin makes clear, it takes money to develop tests and vaccines, money to send people to Zambia or Taiwan or Malaysia or Zimbabwe. And it will take money — Lipkin estimates $10 million a year — to fulfill his vision of producing a portable, fully automated diagnostic system that can function outside a laboratory, empowering clinicians anywhere to take any type of clinical or environmental sample and test it for not just viruses but also bacteria, fungi, and parasites and get a result within hours. Lipkin calls it “Mercury.” If funded, he says, “it will change the whole approach to clinical medicine as well as enable rapid detection of health threats that could become global pandemics.”
Wickiser, meanwhile, has a weather eye on the Oropouche virus, which has been inexorably migrating toward the US.
“We definitely will be dealing with Oropouche virus in the very near future,” Wickiser says. “It’s an arbovirus, meaning it’s carried by insects and is transmitted through insect bites. It came out of the Amazon basin and has spread up throughout the Caribbean. And so much is unknown about it. Is it from a mosquito? A sand flea? There are open questions. But what is absolutely known is that it is moving north, up through Mexico, and has already hit the coast of Florida.”
It turns out that viruses travel regardless of the political winds, and Lipkin’s diagnosis is blunt: “If we don’t find a way to fix this funding problem,” he says, “there will be another pandemic.”
This article appears in the Winter 2025-26 print edition of Columbia Magazine with the title "Night of the Virus Hunters."