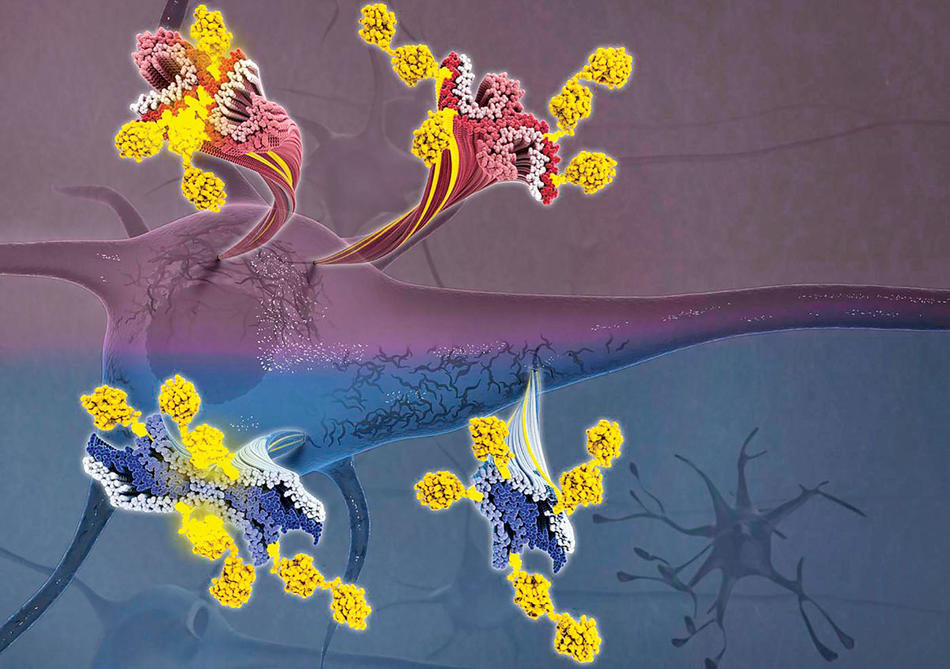
One of the hallmarks of Alzheimer’s disease is that brain cells become clogged with “tau tangles” — long, rope-like bands of tau proteins that obstruct the movement of nutrients within the cells and ultimately cause them to die.
Until now, scientists had never observed the individual proteins up close because they are ten thousand times thinner than a human hair, making them extraordinarily difficult to view, even under powerful microscopes. But a team of neuroscientists at Columbia’s Zuckerman Mind Brain Behavior Institute recently combined two ultra-high-resolution imaging technologies — mass spectrometry and cryo-electron microscopy, the latter a Nobel Prize–winning technology developed in part by Columbia researchers — to examine tau proteins in unprecedented detail and reveal new clues about how and why they accumulate in destructive tangles.
Led by principal investigator Anthony Fitzpatrick, the researchers discovered that tiny molecules located on the proteins’ outer surfaces seem to influence their shape, whether or not they aggregate, and, when they do conjoin, what types of neurocognitive problems result.
The appearance of tau tangles in brain cells is associated not only with Alzheimer’s but also with several other forms of dementia and with chronic traumatic encephalopathy (CTE), a neurodegenerative disease that afflicts many former football players, soldiers, and others who have experienced repeated head injuries. Fitzpatrick says that his team’s research, by identifying key differences between these disorders at the molecular level, could one day enable physicians to anticipate the onset of the conditions before symptoms arise and intervene with novel drug treatments that slow their progress or prevent them altogether.
“Neurodegenerative diseases are among the most complex and distressing class of illnesses,” he says. “But we are building a road map toward successful diagnostics and therapeutics.”