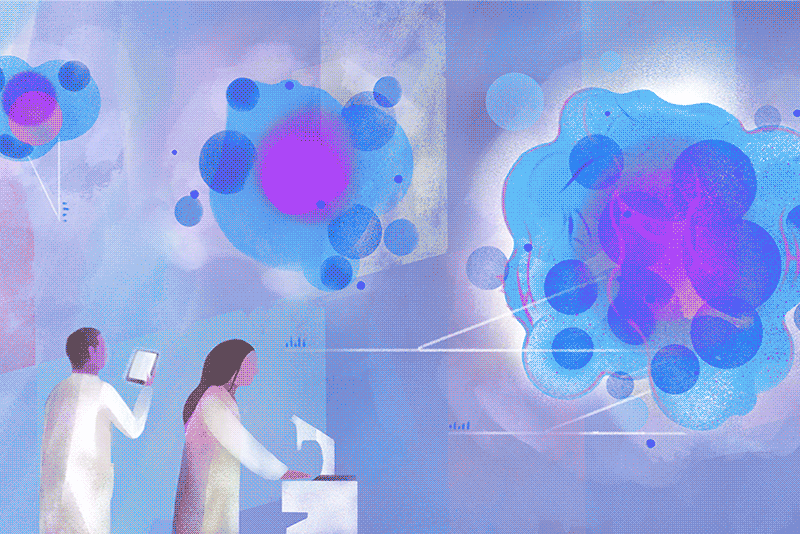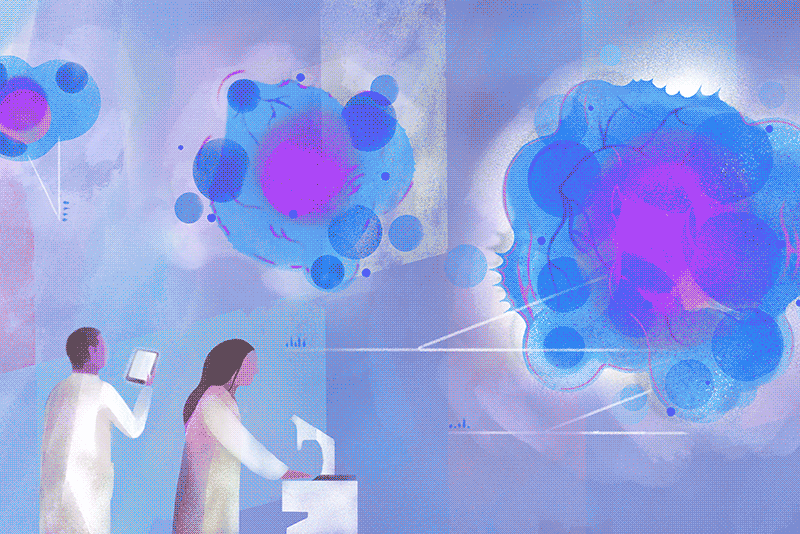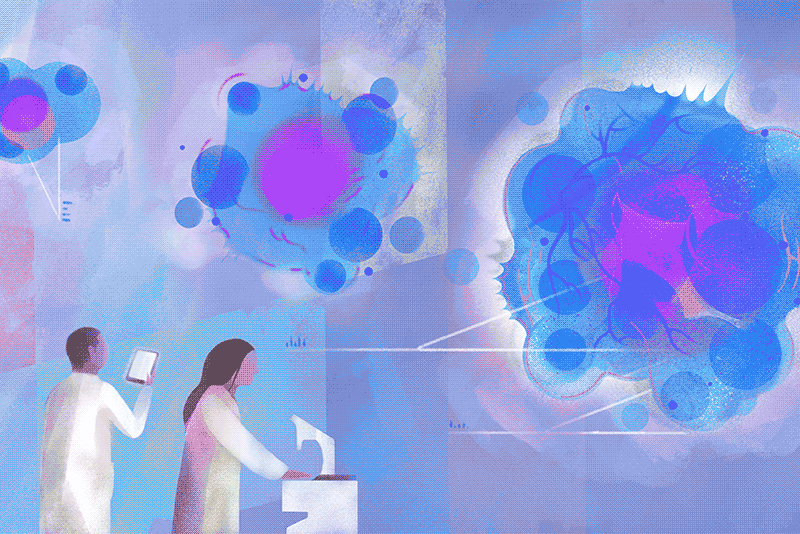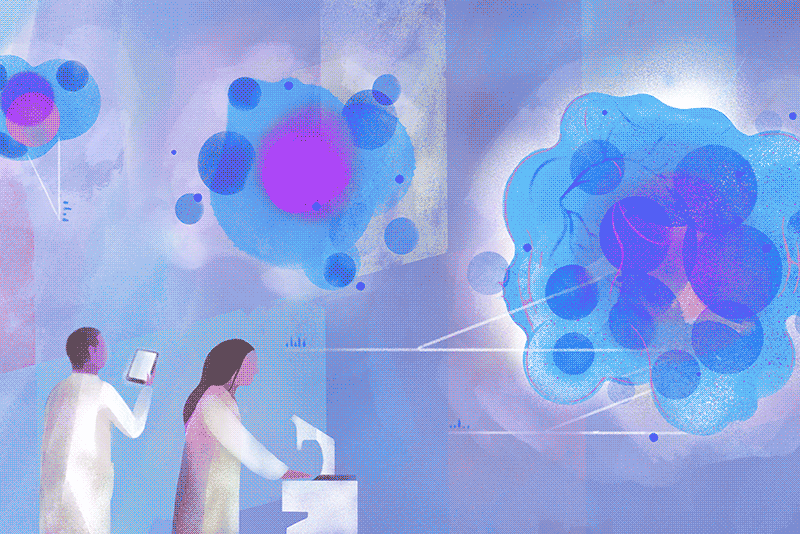
On a cloudy Wednesday morning, Columbia biology chair Brent R. Stockwell peers at a computer in his laboratory at the Northwest Corner Building to see how many cancer cells survived the previous night. He is pleased to find that few have. “What’s interesting is how they died,” he says. “They essentially destroyed themselves, after a nudge from us.”
The idea that cells are programmed to self-destruct under certain conditions has been a cornerstone of biology since the 1970s. That’s when scientists first described apoptosis, a tightly orchestrated process in which old or damaged cells dismantle their internal components, shrink, and chop themselves up into minuscule pieces to be cleared away by the immune system. The excitement that discovery generated is hard to overstate. Until then, most scientists assumed that cells wore out as machines do — gradually and without much rhyme or reason. But if cell death were highly organized and regulated, this suggested that the process could be predicted, influenced, even controlled.
In 2001, Stockwell, then a thirty-year-old research fellow at the Whitehead Institute for Biomedical Research in Cambridge, Massachusetts, became convinced that he was witnessing an altogether different form of programmed cell death. While studying the effects of various chemicals on cancer cells, he noticed that one novel compound caused cells to deteriorate in ways he’d never read about in textbooks. “It wasn’t quite as neat and orderly as apoptosis, but it still seemed to follow a distinct progression,” says Stockwell. The major difference was that the cells he was working with, rather than collapsing in on themselves, swelled up until their outer membranes stretched and leaked. “And then they’d basically explode,” Stockwell says. After repeating the experiment with multiple cell types and observing the same pattern, he felt a jolt of exhilaration. “I was also a bit nervous, to be honest,” he recalls. “I thought, If this really is a new type of cell death, convincing the scientific community is going to be a huge undertaking.”
In fact, validating his discovery would prove to be more difficult and time-consuming than Stockwell could have imagined. Colleagues initially dismissed his results, insisting that he was seeing either a variation of apoptosis or its opposite, necrosis, a chaotic form of cell death that follows sudden injury or trauma. His grant applications were denied. Emails to potential collaborators yielded nothing. “People said that cell death was already thoroughly understood,” Stockwell says, “and to look for other versions was outlandish.”
His luck turned around only after he landed a faculty position at Columbia, with a joint appointment in chemistry and biology, in 2004. “I’d described my plans to explore this phenomenon during my interview, and to my surprise, the science leadership here was enthusiastic,” Stockwell says. With a startup package of funding provided by the University, he set about investigating the strange new form of cell death he thought he’d observed. The initial breakthroughs came a couple of years later, and more have been coming ever since. Stockwell and his colleagues first showed that cells in their experiments were dying as a result of an overaccumulation of oxidized fats in cellular membranes. They next demonstrated that the oxidation was catalyzed by iron stores within cells. Then, in a landmark 2012 paper in the journal Cell, they established that this process was distinct from apoptosis. They named it ferroptosis, from the Latin word for iron, “ferrum,” and the Greek word for falling, “ptosis.”
Today, hundreds of academic laboratories around the world are studying ferroptosis, with more than twenty-two thousand papers published on the topic to date. Researchers across the life sciences are exploring the finer points of its mechanisms, as well as how ferroptosis is involved in medical conditions ranging from cancer and heart disease to autoimmune and neurodegenerative disorders. A new journal devoted to the subject, Ferroptosis and Oxidative Stress, launched this past fall. Its inaugural issue contains the latest discovery by Stockwell’s team, revealing new clues about how ferroptosis could be harnessed to defeat some of the deadliest cancers, including those of the brain, lung, and kidney. Specifically, the Columbia researchers show that cancer cells that manage to evade traditional chemotherapy drugs often do so by undergoing metabolic changes that, paradoxically, make them exceptionally vulnerable to ferroptosis.
“We’re now developing compounds to further destabilize their metabolism and tilt them toward self-destruction,” Stockwell says.
That work is in its early stages, but one experimental compound that the Columbia scientists have designed kills drug-resistant cancer cells in a petri dish, and it is now being tested in animal trials.
Stockwell and his colleagues are also exploring whether a specialized food missing two key amino acids, administered in tandem with their novel compound and traditional chemotherapy drugs, might help to induce ferroptosis in cancer cells.
“Some people say that treating cancer with engineered food that primes cancers to be even more susceptible to ferroptosis is a crazy idea and will never work,” Stockwell says. “But we’re determined to try. It’s like I always tell my students: You can’t play it safe and expect to make a difference. You have to be willing to take risks, to dive into the unexpected, to discover something new.”